Why choose BioGRID for your study?
No matter how complex, time-sensitive, or unique your clinical study is, BioGRID adapts to your needs with precision and flexibility. Discover how our platform can make a difference in your next trial.
Explore how BioGRID adapts to the unique
challenges of diverse clinical studies.

No matter how complex, time-sensitive, or unique your clinical study is, BioGRID adapts to your needs with precision and flexibility. Discover how our platform can make a difference in your next trial.
Accurate, well-structured clinical study documents are essential for regulatory approval and successful study execution.
Our expert writers ensure your protocols and reports are clear, compliant, and tailored to meet regulatory requirements.
Effortlessly manage complex datasets from various studies.
Generate intuitive charts and dashboards for multi-phase trials.
Adjust data inputs as protocols evolve without disrupting workflows.
Track adverse events and therapeutic responses efficiently.
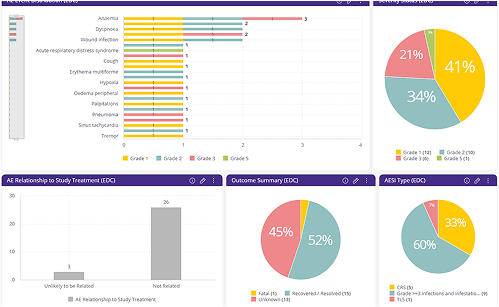
Need for real time dynamic Patient Profiles to drive safety decisions.
Traditional Patient Profiles can take days or weeks to prepare, ultimately leading to delayed decision-making. Time of assessment performed is critical. BioGRID’s streamlined Patient Profiles with dynamic filters and real-time data flow ensure that Patient Profiles is always up to date. Enabling rapid decision-making and ensuring patient safety.
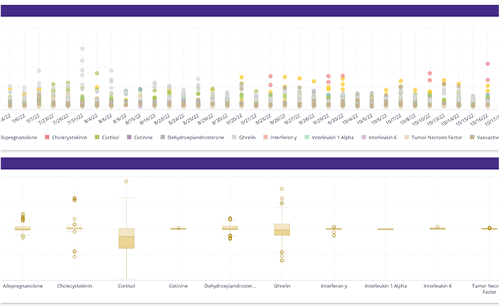
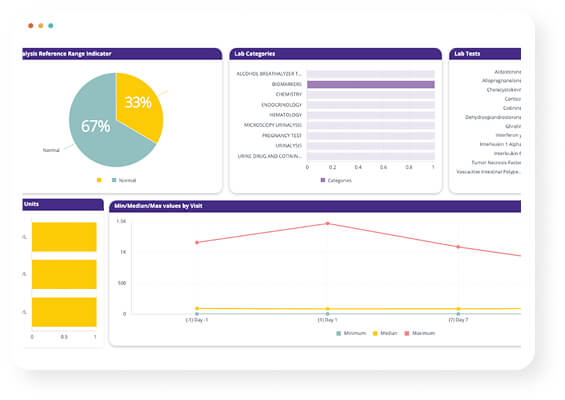
The need to view data across trials (monitory a specific IP) and compounds. To monitor patient safety through adverse events, monitoring patient response, monitoring primary and secondary end points. Not all studies fit conventional templates. For unique trials requiring customized data visualization, BioGRID offers flexible, dynamic dashboard creation.
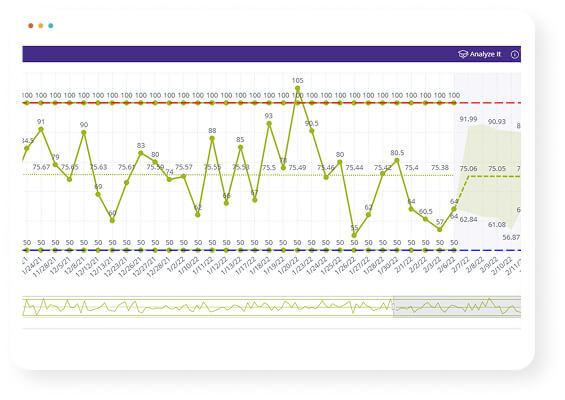
BioGRID includes proactive data surveillance, risk-based quality management (RBQM), and tools to track Key Risk Indicators (KRIs), Potential Discrepancies (PDs), and audit trails—helping ensure full GCP compliance and efficient regulatory review.
BioGRID supports seamless data ingestion from:
We offer:
Yes. BioGRID is designed for scale and performance. It processes large and complex datasets quickly, ensuring smooth analytics and visualization regardless of size.
Yes. BioGRID uses: